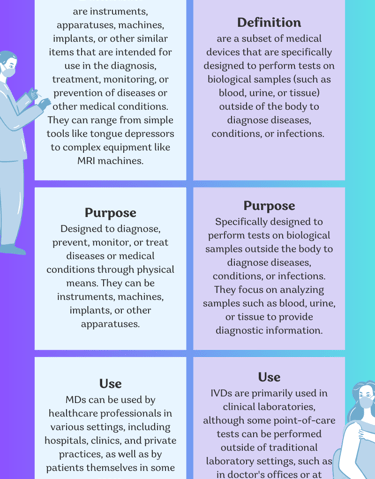
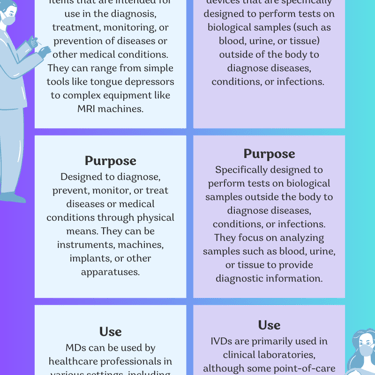
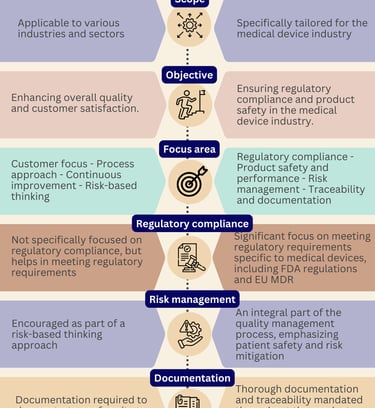
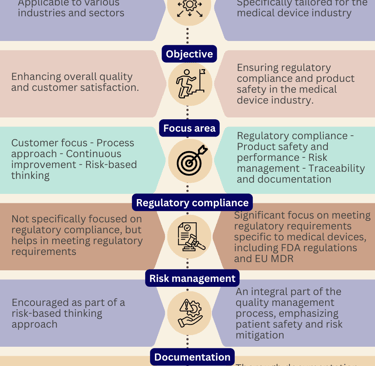
What are the differences between Medical devices and in vitro diagnostics?
Medical devices and in vitro diagnostics (IVDs) are both important components of the healthcare industry, but they serve different purposes and are subject to different regulations.
Are you curious about the differences between the two?
Well, let us break it down for you with this comprehensive infographic overview.